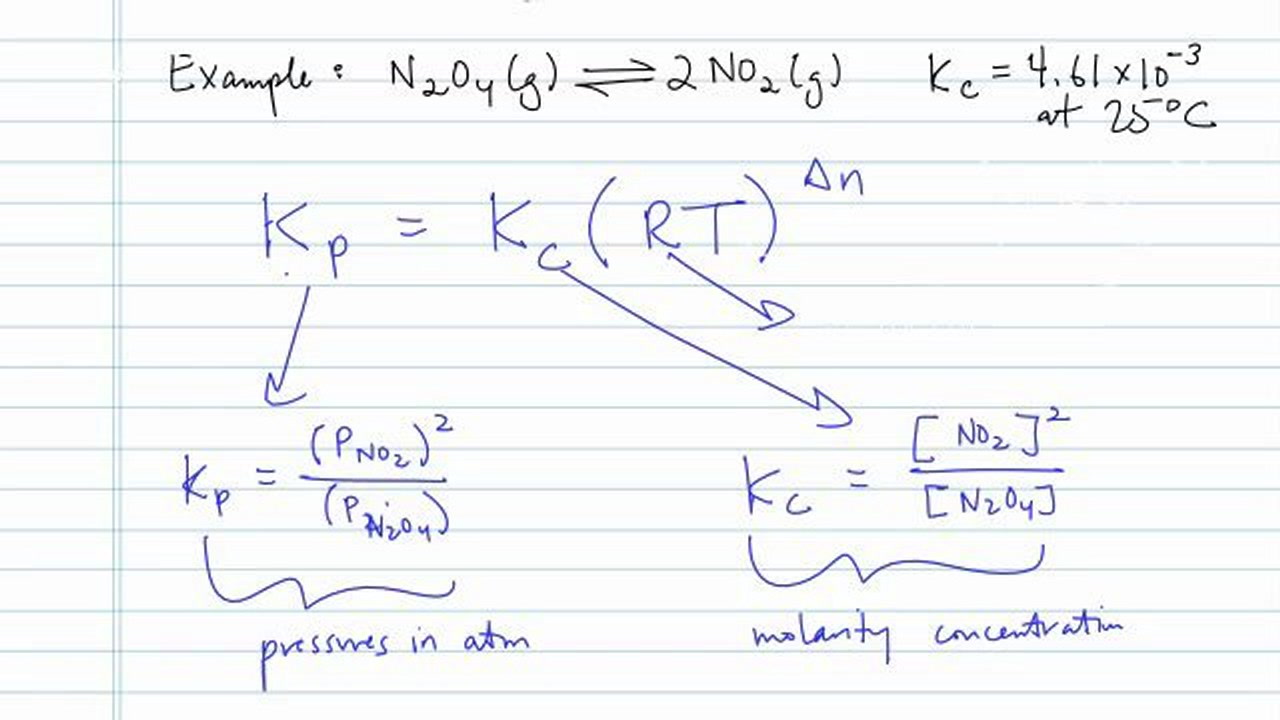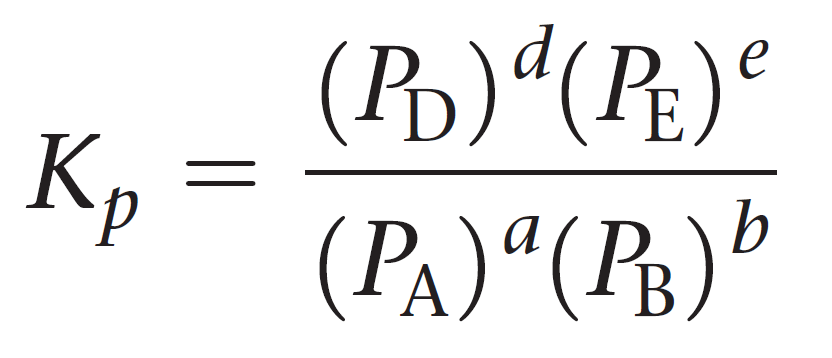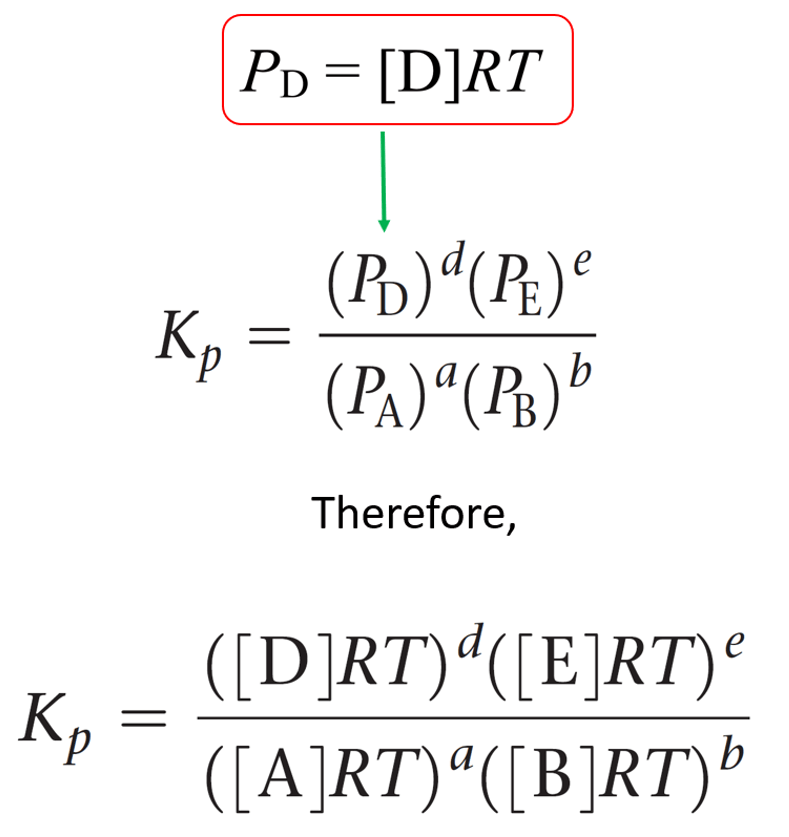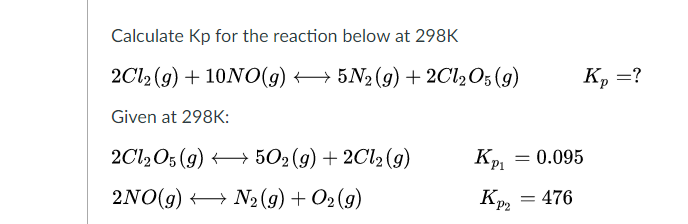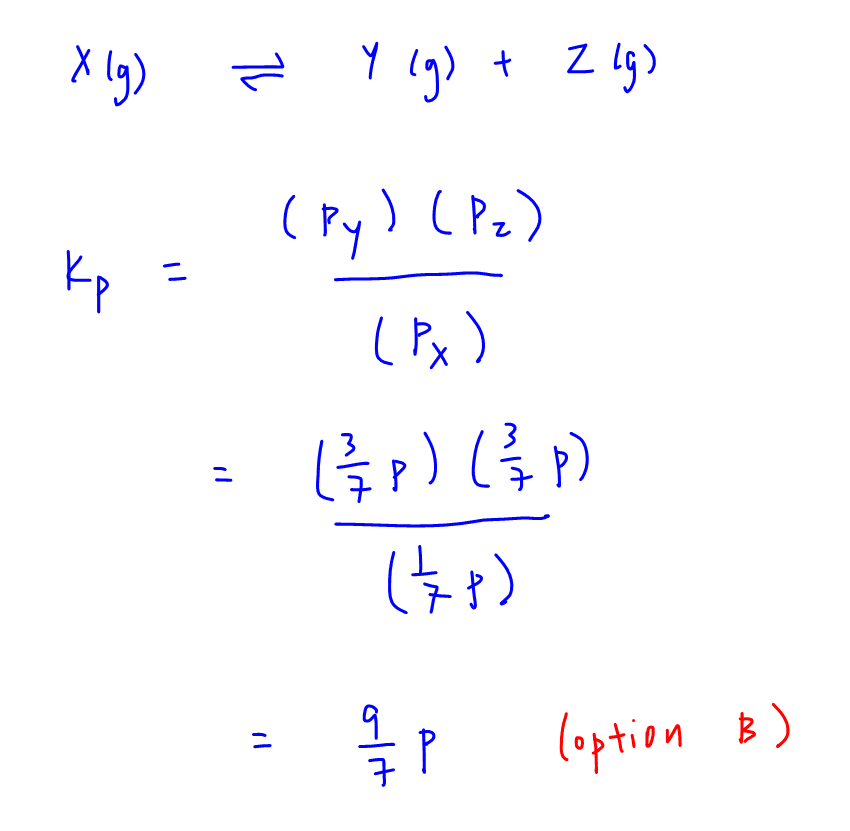
SOLVED: 1. calculate Kp for the reaction COCl2 (g) <—-> CO (g) +Cl2 (g). The equilibrium partial pressure of COCl2 , CO , and Cl2 are 0.20 atm , 0.16 atm, and

Question Video: Calculating the Equilibrium Constant for Partial Pressures Given the Total Pressure and the Percent Dissociation | Nagwa
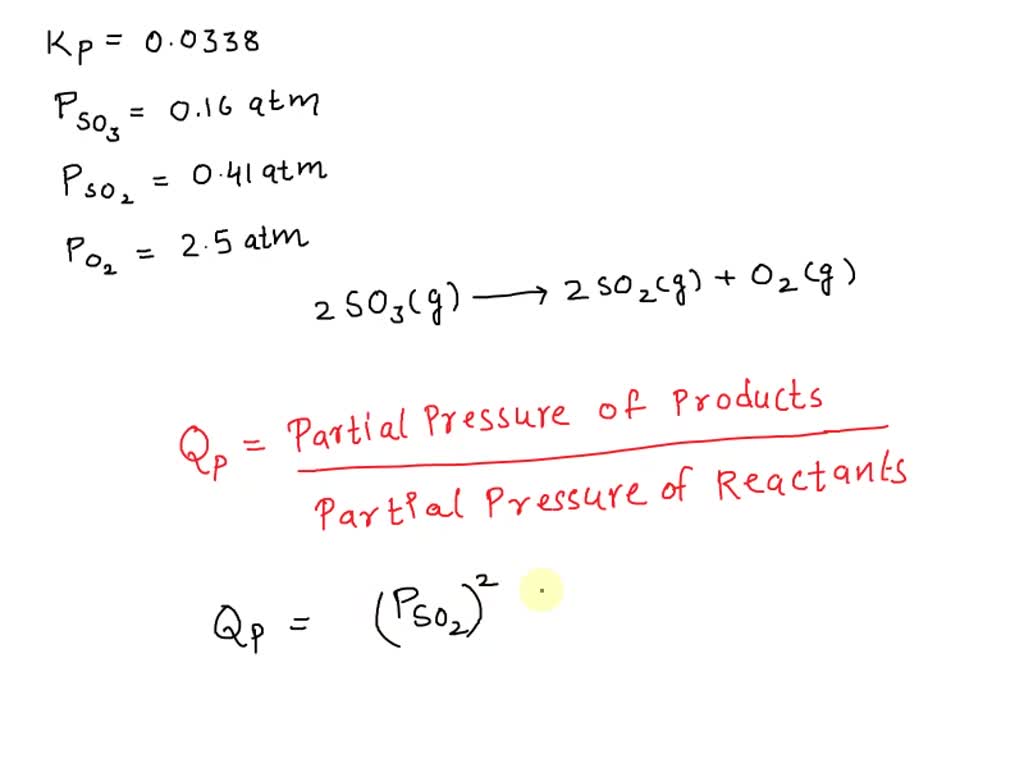
SOLVED: At 1000 K the value of Kp for the reaction 2SO3 (g) → 2SO2 (g) + O2 (g) is 0.0338. Calculate the value of Qp, and predict which direction the reaction

SOLVED:Calculate Kp for each reaction. a. N2O4(g) 2 NO2(g) Kc = 5.9 10-3 (at 298 K) b. N2(g) + 3 H2(g) 2 NH3(g) Kc = 3.7 * 108 (at 298 K) c.
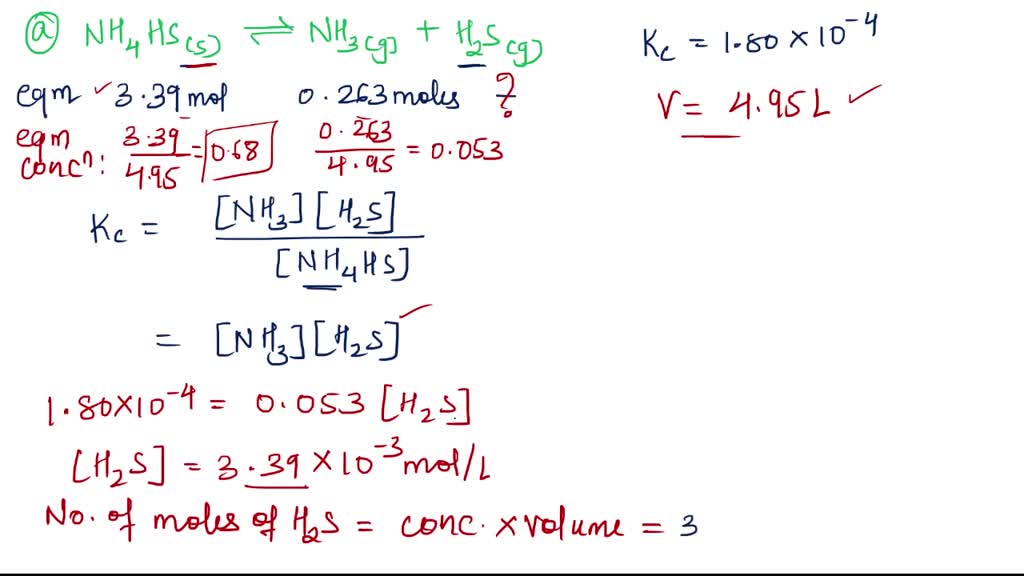
22. Calculate Kp for the equilibrium NH4HS (s) >NH3 (g)+H2S (g) if the total pressure inside the reaction vessel is 1.12 atm at 105^° C